
© 2016, BSM Consulting
6
Modern Glaucoma Surgery
Tube-Shunts
Tube-shunts were developed to reduce the risk of failure from all three potential scarring issues: the hole
scarring shut, the flap scarring to its bed, or the conjunctiva scarring to the sclera. In general, these
devices consist of a long hollow plastic tube attached to a plate-like reservoir. A conjunctival peritomy is
fashioned to give access to the subconjunctival space. The tube end is inserted through the sclera near
the limbus into the anterior chamber, and the reservoir is
attached to the sclera externally approximately 10 mm
behind the limbus. The conjunctiva is then resealed so that
the entire device is located under the conjunctiva (
Figure 3
).
The reservoir is positioned posteriorly so that the eyelids do
not rub the conjunctiva against it with every blink. However,
the lids do rub the conjunctiva against the tube with every
blink. Over time, this can lead to erosion of the conjunctiva
over the tube, which causes pain and represents a high risk
for infection since bacteria can get into the subconjunctival
space and can find their way into the eye. To prevent this, a
small piece of donor sclera or pericardium (the outside
lining of the heart) can be sewn over the tube but under the
conjunctiva, preventing rubbing of these two and reducing
the risk of erosion. The tube props the hole open preventing
it from scarring shut. There is no scleral flap to scar to its
base; the reservoir essentially props open the
subconjunctival space so that the conjunctiva cannot scar
to the sclera. This sounds ideal, but as will be discussed in
the following paragraphs, these procedures often fail due to
scarring as well.
There are two main types of tube-shunts: valved and non-valved. Valved devices (of which the Ahmed
implant is the most common) have a built-in flow restrictor in the tube to limit the rate of aqueous exiting
the eye in order to minimize the risk of hypotony. Non-valved devices (of which the Baerveldt implant is
the most common) have no flow restrictor. In the long term, they rely on the formation of a fibrous capsule
of scar tissue around the reservoir to provide adequate resistance to aqueous outflow to avoid
postoperative hypotony. Clearly, this is a delicate balance: enough scarring to slow aqueous flow and
prevent hypotony but not enough to completely block aqueous flow causing failure of the procedure (this
is how these procedures can fail despite addressing all three of the typical sites of scarring described
previosuly). Short-term, before the capsule forms, there is no resistance to outflow and hypotony is a very
real concern. To prevent this temporarily, the surgeon can tie a dissolvable suture tightly around the
outside of the tube to crimp it shut. The suture typically dissolves in four to six weeks, when the capsule
has formed. This means that the operation will not be effective until the suture dissolves, so the patient
will likely continue to need medications for IOP control postoperatively until this occurs. The surgeon can
also place a thick suture inside the tube to basically plug it up, with one end coming out through the
reservoir end. This “loose” end can be secured in such a way that the surgeon can later grasp it and pull it
out to commence aqueous outflow once the capsule has formed.
TRABECULAR BYPASS (NO-BLEB) PROCEDURES
The three procedures described in the last section involve creating a drain through the full thickness of
the eye wall with aqueous being shunted to the subconjunctival space, forming a bleb. The presence of a
bleb conveys a life-long risk of bleb-related complications, including leaks, blebitis (infection limited to the
bleb), and endophthalmitis (infection inside the eyeball). The procedures described in the following
material were developed to achieve aqueous outflow
without
the formation of a bleb, with the goal of
creating a safer procedure for glaucoma surgery.
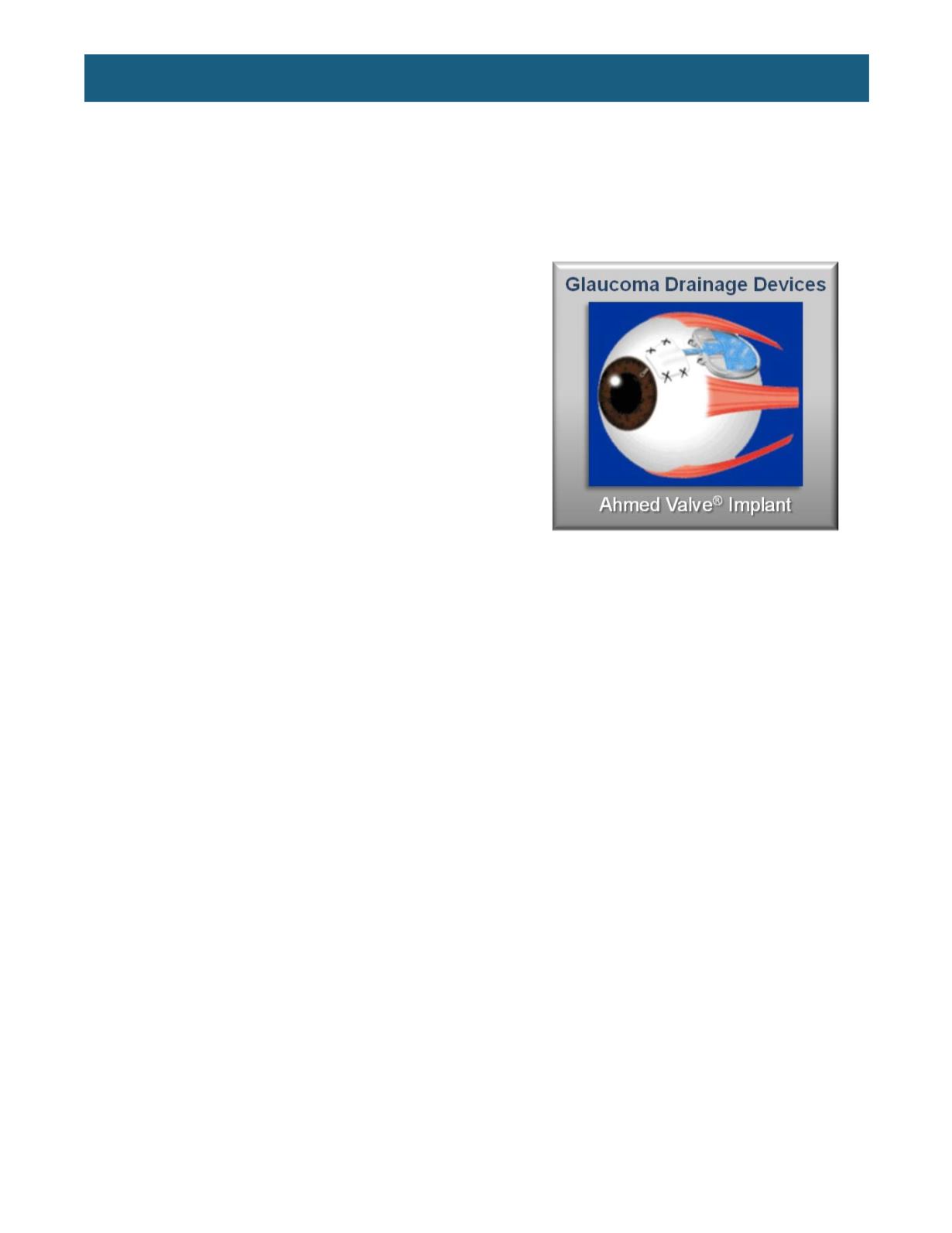
Figure 3.
An implanted Ahmed valve.
(From
http://telemedicine.orbis.org/bins/content_page.asp?cid=735-2858-4396-2862-
12134-2863-3994)














